Cardiac Safety Services Market Size to Reach USD 1,888.34 Million by 2032, Growing at 11.48% CAGR
Rising Cardiovascular Disease Incidences, Clinical Trials, and Regulatory Demands Propel Cardiac Safety Services Market Growth
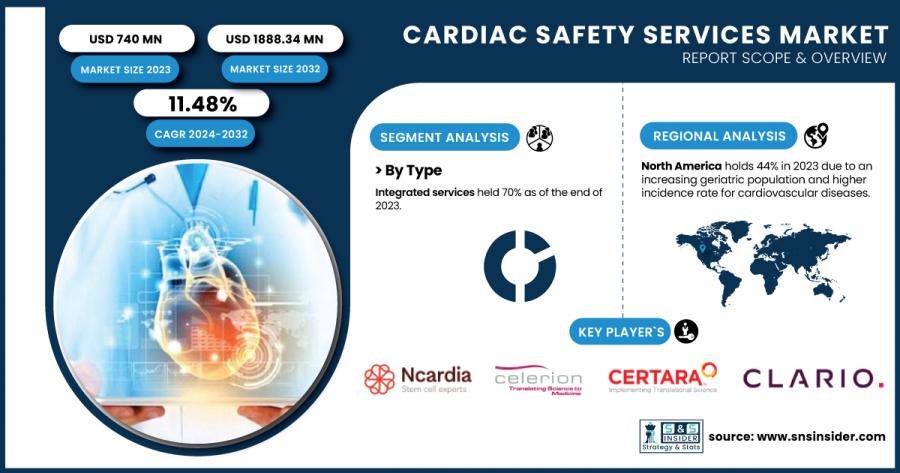
AUSTIN, TX, UNITED STATES, January 30, 2025 /EINPresswire.com/ -- According to Research by SNS Insider, the global cardiac safety services market was valued at USD 740 million in 2023 and is expected to grow at a CAGR of 11.48%, reaching USD 1,888.34 million by 2032
The cardiac safety services market is witnessing strong growth due to a combination of rising cardiovascular disease prevalence
The growing emphasis on patient safety in clinical trials, and regulatory mandates that require comprehensive cardiac testing. As drug safety and efficacy are becoming a more pressing issue, pharmaceutical companies and contract research organizations (CROs) are seeking specialized service providers to which they can outsource cardiac safety testing. Cardiovascular diseases are one of the leading causes of reason in the world which acts as the primary problem driving market. According to the World Health Organization (WHO), cardiovascular diseases are the leading cause of death worldwide, accounting for nearly 32% of all global deaths in 2023. The ever-increasing need for successful therapies, it is also essential that drugs and treatments are safe for patients with cardiovascular diseases. As a result, there is rising demand for these cardiac safety services, which are critical for clinical trials as they will help in monitoring cardiac health and drug-induced cardiac disorders.
In addition, regulatory agencies are implementing tougher guidelines for evaluating new drugs and medical devices relative to cardiovascular safety (e.g., U.S. Food and Drug Administration [FDA], European Medicines Agency [EMA]). For example, the guidance on cardiac safety assessment of drug products to be audited submitted in 2022 by the FDA addresses how non-cardiac drugs have QT prolongation and other cardiovascular data that should be used in clinical trial audiences. The increasing regulatory pressure is leading the pharmaceutical companies to rely on the cardiac safety services. However, emerging technologies including electrocardiogram (ECG), Holter monitoring, and cardiac bio-markers are further improving the precision and efficiency of cardiac safety testing. In addition, artificial intelligence (AI), coupled with enhanced predictive analytics via machine learning is being harnessed for advance efforts in cardiac safety testing to facilitate early identification of potential cardiotoxicity emerging over the course of development through the clinical trial phase.
Get a Free Sample Report of Cardiac Safety Services Market @ https://www.snsinsider.com/sample-request/4378
Key Players in Cardiac Safety Services Market
• Ncardia AG
• Celerion
• Certara
• Clario
• Richmond Pharmacology
• Koninklijke Philips N.V.(BioTelemetry)
• Laboratory Corporation of America Holdings
• Medpace
• Biotrial
• PhysioStim
Segment Analysis
By Type
In 2023, this market was dominated by integrated services segment with its 70% share in total revenue. Integrated cardiac safety services provide end-to-end solutions that include clinical trial design, patient monitoring, data collection, analysis, and reporting. These services are gaining popularity owing to their convenience as they provide a streamlined operation for the pharmaceutical and biopharmaceutical companies. They also keep the clinical trials quicker, and regulatory-compliant by providing integrated solutions.
By End-User
In 2023, the cardiac safety services market was dominated by pharmaceutical and biopharmaceutical companies with the revenue share of 45%. This is mainly because of the increasing requirement of a secure and efficient drug development process. As the world market for drugs, especially for heart diseases, widens, pharmaceutical companies can be seen pouring money to obtain the best safety profiles for their products. Hence, these companies are engaging specialized players that provides cardiac safety services over others to fulfil regulatory requisites and reduce the chances of adverse cardiac events.
Contract research organizations (CROs) and academic institutions are also significant end-users, though their market share is relatively smaller compared to pharmaceutical companies. In particular, CROs which conduct the clinical trials on behalf of the pharma and biopharma companies are increasingly depending on cardiac safety service providers to manage the trial data and maintain compliance with global standards.
Cardiac Safety Services Market Segmentation
By Type
• Integrated
• Stand-alone
By Service
• ECG/Holter Measurement
• Blood Pressure Measurement
• Cardiovascular Imaging
• Thorough QT Studies
• Others
By End User
• Pharmaceutical and Biopharmaceutical Companies
• Contract Research Organizations
• Others
Need any customization research on Cardiac Safety Services Market, Enquire Now @ https://www.snsinsider.com/enquiry/4378
Regional Analysis
North America accounted for the largest share 44% of the cardiac safety services market in 2023. This is due to the developed healthcare system in the US and the demanding regulatory criteria maintaining a high bar for clinical trials. Rigorous cardiac safety assessments have become essential in drug development processes partly due to the role of the U.S. food and drug administration (FDA) and other regulatory agencies. In addition, the U.S. contributes a large share of international research and development (R&D) expenditures. With over USD 600 million allocated to cardiovascular research in 2023, the National Institutes of Health (NIH) funding of these mechanisms of drug action is an example of government attention to improving patient safety whilst enabling drug development. As a result of the increasing prevalence of cardiovascular diseases among the region, it is estimated, that the demand for specialized cardiac safety service is also increasing.
Asia-Pacific is the fastest growing region for cardiac safety services market. As nations, such as China, India, and Japan, invest heavily in healthcare infrastructure and grow their clinical trial capacity, they are seeing a rise in clinical trials, particularly of new cardiovascular drugs and treatments. The Indian government has placed greater importance on healthcare reforms and clinical trials, as highlighted through the Ayushman Bharat initiative that emphasises on expanding access to comprehensive healthcare services, including specialized cardiac services. At the same time, rapid rise in the pharmaceutical industry in the region is inducing the demand for cardiac safety services. Healthcare expenditure in the Asia and Pacific region, driven by an ageing population and increasing chronic disease incidence, is projected to increase by 40% between 2015 and 2025, according to the Asian Development Bank (ADB). This offers a huge revenue generation opportunity for cardiac safety services market in the region.
Recent Developments
• In March 2024, Labcorp Drug Development launched an advanced cardiac safety monitoring solution that integrates real-time data collection and AI-driven analytics. This technology aims to provide faster, more accurate insights into the cardiovascular effects of investigational drugs, improving clinical trial efficiency and patient safety.
• In 2023, Novartis expanded its partnership with a leading contract research organization (CRO) to enhance its drug safety monitoring processes. As part of the collaboration, the company will increase its use of cardiac safety services to meet regulatory requirements and improve patient safety for its new cardiovascular drug pipeline.
Buy Full Research Report on Cardiac Safety Services Market 2024-2032 @ https://www.snsinsider.com/checkout/4378
Table of Contents – Major Key Points
1. Introduction
2. Executive Summary
3. Research Methodology
4. Market Dynamics Impact Analysis
5. Statistical Insights and Trends Reporting
6. Competitive Landscape
7. Cardiac Safety Services Market by Type
8. Cardiac Safety Services Market by Service
9. Cardiac Safety Services Market by End User
10. Regional Analysis
11. Company Profiles
12. Use Cases and Best Practices
13. Conclusion
Speak with Our Expert Analyst Today to Gain Deeper Insights @ https://www.snsinsider.com/request-analyst/4378
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Browse More Insights:
Virtual Clinical Trials Market Size & Growth Report
Biopharmaceutical Size & Growth Report
Akash Anand
SNS Insider Pvt. Ltd
415-230-0044
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release