ONWARD's FDA-Authorized ARC-EX System Restores Hand Strength and Sensation in Spinal Cord Injury Patients
ONWARD's ARC-EX System uses spinal cord stimulation to boost strength and mobility, offering new hope for Veterans and those with spinal cord injuries.
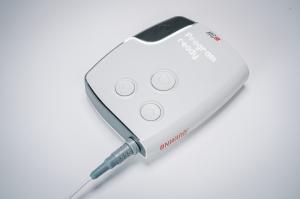
The ARC-EX System delivers targeted, programmed stimulation of the spinal cord to restore hand strength and sensation in people with SCI. This first-of-its-kind therapy represents a significant advancement in the treatment options available to Veterans and others living with SCI. It was authorized for commercial sale in the US by the FDA in December 2024.
"Adding the ARC-EX System to two major US federal government purchasing platforms is an important step toward making our therapy broadly available to US military Veterans living with spinal cord injury," said Dave Marver, CEO, ONWARD Medical. "The VA serves a significant percentage of Americans with spinal cord injury, and establishing these procurement channels soon after FDA authorization demonstrates our commitment to ensuring federal healthcare providers have rapid access to the latest technology."
For questions about the ARC-EX System and its availability in the United States, visit this webform.
To learn more about ONWARD Medical’s commitment to partnering with the SCI Community to develop innovative solutions for restoring movement, function, and independence after spinal cord injury, please visit ONWD.com.
"We’re excited to see the ARC-EX System receive FDA authorization. This innovative therapy has the potential to significantly improve strength and mobility for individuals with SCI." said Chris Lovell, Major, USMC (Ret.), CEO of Lovell Government Services.
About Lovell Government Services
Lovell Government Services has been a trusted SDVOSB vendor since 2013 with a proven track record of successfully introducing suppliers to the government market. Lovell is a three-time Inc. 5000 honoree and leader in the federal space. They partner with medical and pharmaceutical companies looking to better serve Veteran and military patient populations, increase their federal revenue stream, and win government contracts.
For more information, visit www.lovellgov.com
About ONWARD Medical
ONWARD Medical is a medical technology company creating therapies to restore movement, function, and independence in people with SCI and other movement disabilities. Building on more than a decade of scientific discovery, preclinical research, and clinical studies conducted at leading hospitals, rehabilitation clinics, and neuroscience laboratories, the Company has developed ARC Therapy, which has been awarded ten Breakthrough Device Designations from the US Food and Drug Administration (FDA). The Company’s ARC-EX System is now cleared for commercial sale in the US. In addition, the Company is developing an investigational implantable system called ARC-IM with and without an implanted brain-computer interface (BCI).
Headquartered in the Netherlands, the Company has a Science and Engineering Center in Switzerland and a US office in Boston, Massachusetts. The Company is listed on Euronext Paris, Brussels, and Amsterdam (ticker: ONWD).
For more information, visit ONWD.com and connect with us on LinkedIn and YouTube.
For Media Inquiries:
media@onwd.com
For Investor Inquiries:
Investors@onwd.com
Disclaimer
Certain statements, beliefs, and opinions in this press release are forward-looking, which reflect the Company’s or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve several risks, uncertainties, and assumptions that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties, and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, delays in regulatory approvals, changes in demand, competition, and technology, can cause actual events, performance, or results to differ significantly from any anticipated development. Forward-looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions, or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.
ARC-EX Indication for Use (US): The ARC-EX System is intended to deliver programmed, transcutaneous electrical spinal cord stimulation in conjunction with functional task practice in the clinic to improve hand sensation and strength in individuals between 18 and 75 years old that present with a chronic, non-progressive neurological deficit resulting from an incomplete spinal cord injury (C2-C8 inclusive).
Other Investigational Products: All other ONWARD Medical devices and therapies including ARC-IM and ARC-BCI are investigational and not available for commercial use.
Trademarks: ONWARD, ARC-EX, ARC-IM, ARC-BCI, and the stylized O-Logo are proprietary and registered trademarks of ONWARD Medical. Unauthorized use is strictly prohibited.
For Media Inquiries:
Ryan Camarra
Lovell Government Services
+1 850-684-1867
email us here
Distribution channels: Healthcare & Pharmaceuticals Industry, Military Industry, Science, Sports, Fitness & Recreation, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release